Mountain Science – preliminary results 1
I’ve recently been looking over the data I took while on expedition to Putha Hiunchuli in Nepal. The plan was to take some data and do some experiments looking at basic science in the mountains, particularly around issues that affect climbers. The main topics were:
- reduced air pressure at altitude
- increased ultraviolet radiation at altitude
- reduced temperature at altitude
Alongside Anturus I am making some videos as educational resources, but here are some first results.
Air Pressure
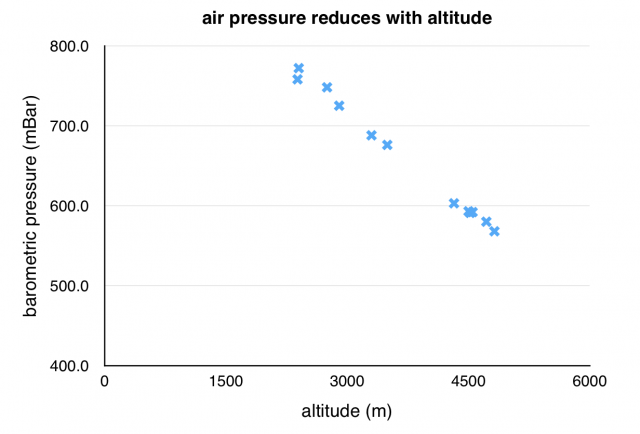
Air pressure reduces with altitude. That is, the mass of air pushing down on us is less as we go higher.
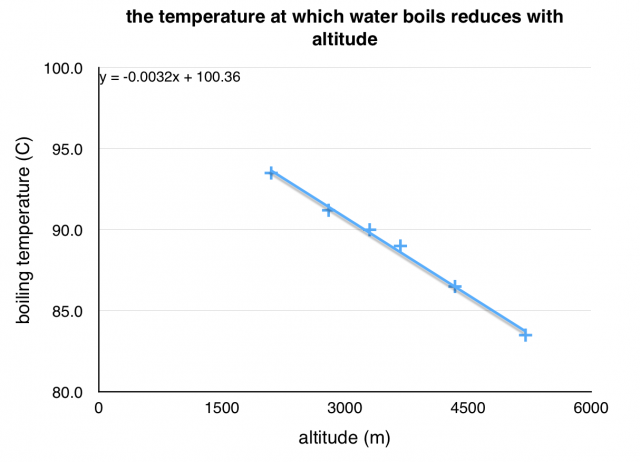
To look at the effect of this, I did something we do every day – I boiled some water. We all know that at sea level water boils at 100 ºC, but look how this changes as we go higher.
Above is a picture of me with my Jetboil and my data logger up at 3675m measuring the temperature at which the water was boiling.
The temperature at which water boils reduces with altitude because the air pressure reduces.
Liquids turn into gases when they are heated because that heat energy is transferred to the particles of the liquid and causes them to move faster. When they are moving fast enough, the particles near the surface can escape into the air and become a gas. When a lot of liquid particles are escaping and the temperature stops rising we say the liquid is boiling.
However, in the air there are air particles that are pushing down lightly on the liquid and making it harder for the liquid particles to escape. A lower air pressure means there are fewer of these air particles pushing down on the surface of the liquid, so it is easier for the liquid particles to escape, so we don’t need to apply so much heat. Therefore the water boils at a lower temperature.
The way that water boils at a lower temperature is a demonstration of the affect of this lower air pressure. It can be a problem for climbers because they need to rehydrate their instant mountain food using boiling water. Water at 80 ºC doesn’t rehydrate food properly.
However, this reduced air pressure at altitude has a much more serious effect on the climber’s body – reduced oxygen. I’ll show some results on this in the next blog.
Thanks to my sponsors: